hydrate formation chart
Sum the amounts in steps 4 5 and 6 for the total methanol needed. Chart gives hydrate formation temperature for natural gas.
What Is The Impact Of Water Content On The Dew Point And Hydrate Phase Behavior Campbell Tip Of The Month
And sufficient quantities of water and formation time.
. Both sets of data were examined here and no distinction is made between the two. Download scientific diagram Chart of hydrate formation. 2 and the data in Table 5.
Computer solutions are generally more accurate and have applicability. Kartz Towler Bahadori and New Correlation are used to predict the. Baillie C and Wichert E.
Hydrate formation curve in Excel Prode Properties 119 includes a utility which allows to calculate the hydrate formation curve directly in Microsoft Excel the distribution files include a Excel page hydratexls from this page one can define composition including all common hydrate formers C1 Methane C2 Ethane C3 Propane C4 n Butane C4 i Butane N2 Nitrogen. Charts for predicting the pressure to which natural gases may be expandedwithout hydrate formation have been prepared for gases of even gravityPressure-temperature curves for hydrate formation were established for gaseshaving gravities from 06 to 10. This was due to.
These curves and the thermal behavior of thegases during free and adiabatic expansion were used to. AbstractNote A graphical method has been developed that permits the estimation of the hydrate forming temperature at pressures in the range of 100 psia to 4000. The classic method as described is to add products which are thermodynamic hydrate inhibitors eg alcohols such as methanol or glycols.
Hydrate equilibrium and the understanding of natural gas hydrate formation accumulation and destabilization in nature. The study and re-search on hydrate became of interest to the oil and gas industry in 1934 when the first pipeline blockage was observed by Hamrnerschmidt. The knowledge of the dynamics of hydrate formation and accumulation of hydrate crystals is important in determining the parameters for mass production of gas hydrates and in understanding plug conditions in the.
There is no single indicator that gives the best warning of hydrate formation but pressure drop is the most common. In this post I want to share how to predict hydrate formation. There are at least two methods used to predict hydrate formation.
The experimental data specifications including ethylene glycol concentration gas specific gravity. Mhyd ρwater αwaterρgas αgas 8 To incorporate the rheological effects of the increase of suspension in the solution the following relationship was used 1015. Hydrate formation does not become a threat to pipe flow unless the agglomerates and hydrates formed at the interface start forming bridges.
It should be used for first approximation only. The formation of hydrate has two major steps-nucleation and growth and is a time-dependent process. Hydrate formation is modelled by mass transfer equations for both the phases ie.
Petroleum Engineering View metadata. However this solution is very expensive as the quantity of inhibitors to be added may reach 1040 of the water content. In such cases blockage occurs where the small accumulations of hydrates adhere to the walls and begin to bridge and reduce flow.
µslurry αwater1 αhyd 25 9 4 Results and discussion The effect of. With time the formation deposition and adsorption of hydrates on the internal surfaces of pipes wellbore processing facilities and piping components restricts and disrupts hydrocarbon production and in worst cases the production. PREDICTION OF GAS-HYDRATE FORMATION CONDITIONS IN PRODUCTION AND SURFACE FACILITIES A Thesis by SHARAREH AMERIPOUR Submitted to the Office of Graduate Studies of Texas AM University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE August 2005 Major Subject.
The formation of a hydrate requires three conditions. Water and gas by the following simplified relationship. Start by calculating the gas gravity γg using Eq.
Hydrate formation is unfavorable in most cases since it represents a challenge for flow assurance and production system integrity. The maximum H 2S concentration in the study of. Katz 1954 examined the hydrate formation conditions for mixtures of hydrogen sulfide and methane.
The method is valid up to pressures of approximately 1100-1500 psia depending on the composition of the gas. Pressure-temperature correlation is used when composition of stream is not known. The presence of hydrate formers such as CH4 C2H4 CO2 and H2S.
This bridging can even tually shut down the en tire pipeline or field until the hydrates have been. The hydrate formation temperatures with inhibitor for nine natural gas samples with specific gravities in the range of 055 to 067 were calculated using the above-mentioned correlations and the results were compared with experimental data. The data represent a combination of experimentally measured compositions and inferred compositions see original paper for details.
Pressure-temperature curve for predicting hydrate formation. Gas Hydrate was discovered by Sir Humphrey Day in 1810. Hydrate formation is notorious in the gas and oil industry.
Experimental and Modeling Studies on the Prediction of Gas Hydrate Formation On. Rate of Hydrate Formation. Step 1Calculate hydrate formation conditions using the gas gravity chart.
An example of the method is shown in the GPSA Engineering Data Book 1994 on page 20-11. At γg 0704 the gas gravity chart shows the hydrate temperature to be 65F at 1050 psia. This paper aims to predict the Hydrate Formation Temperature at certain pressure by using some of HFT calculation methods.
Hydrate Formation in Multiphase Flow in Pipe By Seng Sook Harn 12842 Interim Report submitted in partial fulfillment of The requirements for the Bachelor of Engineering Hons Mechanical Engineering AUGUST 2013 Universiti Teknologi PETRONAS Bandar Seri Iskandar 31750 Tronoh Perak Darul Ridzuan CORE Metadata citation and similar papers at coreacuk. Low temperature and high pressure. The K-chart method is used to predict the temperature or pressure at which hydrates form in a gas.
What Is The Impact Of Light Hydrocarbons On The Natural Gas Hydrate Formation Conditions Campbell Tip Of The Month

Hydrate Formation Envelope Download Scientific Diagram
Sour Gas Hydrate Formation Phase Behavior Campbell Tip Of The Month
What Is The Impact Of Water Content On The Dew Point And Hydrate Phase Behavior Campbell Tip Of The Month
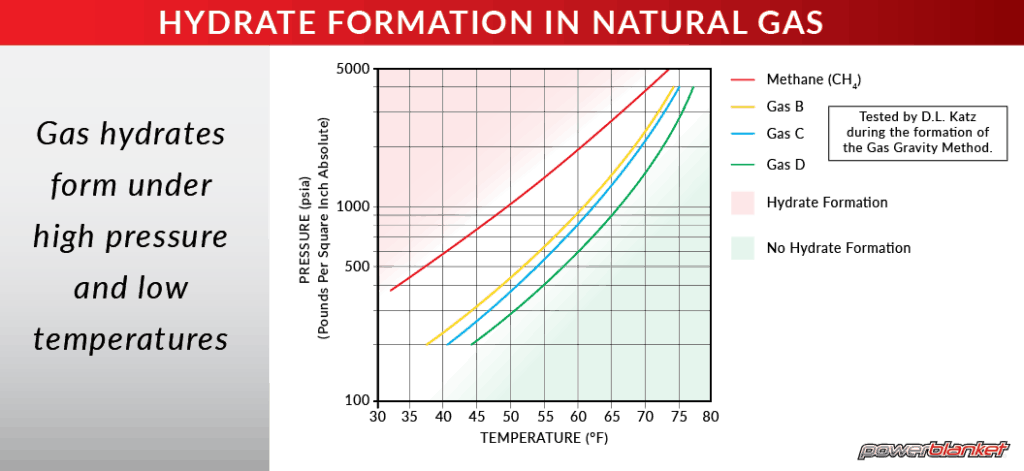
Preventing Gas Hydrate Formation Powerblanket
Sour Gas Hydrate Formation Phase Behavior Campbell Tip Of The Month

Possible Wellbore Problems During Well Kill Operation

Saturated Water Content An Overview Sciencedirect Topics
What Is The Impact Of Light Hydrocarbons On The Natural Gas Hydrate Formation Conditions Campbell Tip Of The Month

Hydrate Formation Curve An Overview Sciencedirect Topics

Hydrate Formation Curve An Overview Sciencedirect Topics
What Is The Impact Of Light Hydrocarbons On The Natural Gas Hydrate Formation Conditions Campbell Tip Of The Month
Predict Gas Hydrate Formation Temperature With A Simple Correlation

Hydrate Formation In Gas Systems Neutrium
Hydrate Formation Envelope Download Scientific Diagram

Hydrate Formation Temperature An Overview Sciencedirect Topics
What Is The Impact Of Nitrogen On The Natural Gas Hydrate Formation Conditions Campbell Tip Of The Month

Hydrate Formation Curve An Overview Sciencedirect Topics
Sour Gas Hydrate Formation Phase Behavior Campbell Tip Of The Month
0 Response to "hydrate formation chart"
Post a Comment